API Development
At GMI We collobarate with one of the best physical property characterization laboratory in the industry.
- We committed to minimizing impact to the environment, reduce process variation, eliminate waste and improve precess effectriveness.
- We developed APIs with high scientific and engineering capability that will drive down your cost of goods.
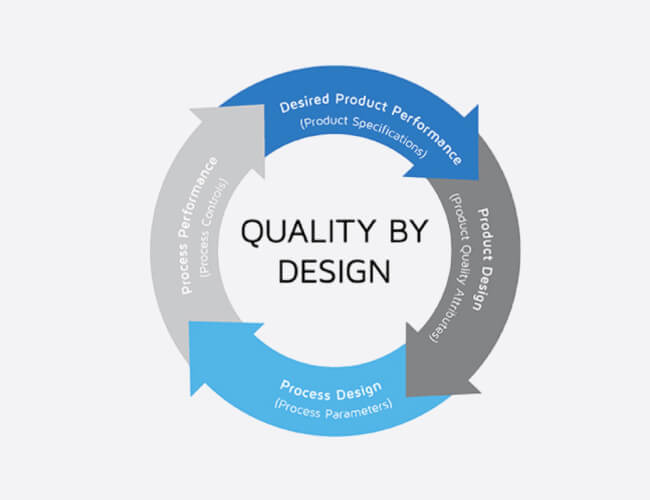
Drug Formulation Development
At GMI We have developed formulation on the basis of QbD (Quality By Design) concept.
- GMI is collaboratively developing a portfolio of generic prescription products in various dosage forms covering a broad range of therapeutic categories with addressing formulation challenges in Stability, Solubility and Bioavailability with focus on Quality, Speed and Cost-effectiveness.
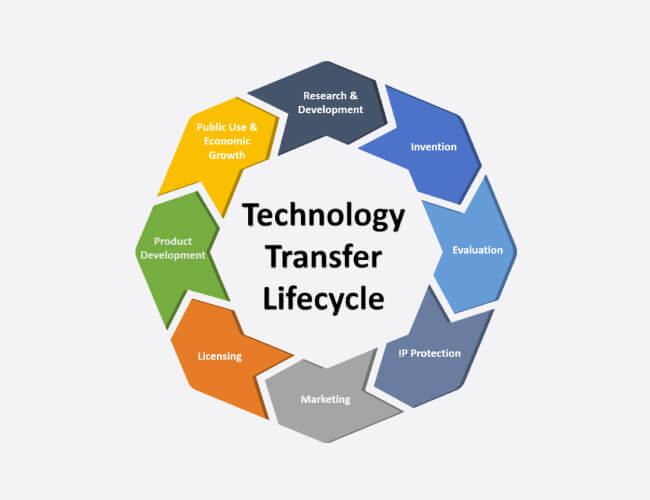
Tech Transfer
GMI is provide expertise in tech transfer by identifying major risk to the process, Critical Process Parameter (CPPs) and facility Mapping.
- We support tech transfer by knowledge transfer about equipment, procedures, competencies, roles & responsibility, regulatory and training requirements.
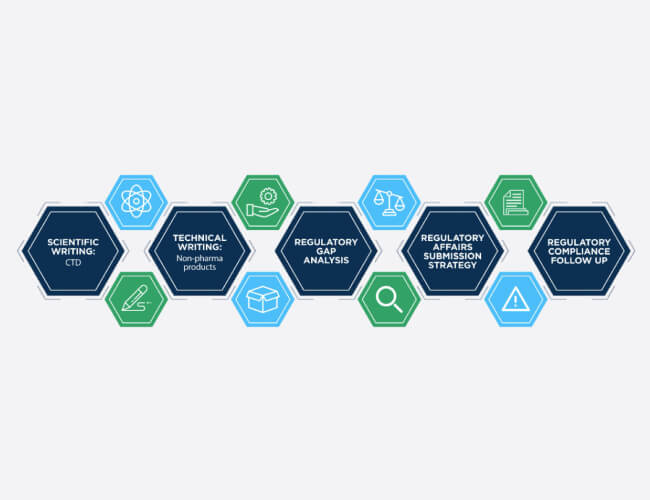
Regulatory Support
The mission of GMI is to provide guidance on regulatory requirement for global market.
- We provide single point of contact to assist with registration strategies for products and processes.
- GMI can provide comprehensive evaluation to support our cleint from initail retulatory requirement through final health authority approval.


